.png?width=576&height=265&name=Blog%20Post_%20New%20Timeline%20eAF%20(4).png)
Updated timeline for the use of eAFs for variations in human drugs
The European Medicines Agency (EMA) recently released an updated timeline with related milestones for using web-based, electronic application forms (eAFs) for variants of medicines for human use approved through both central and national procedures.
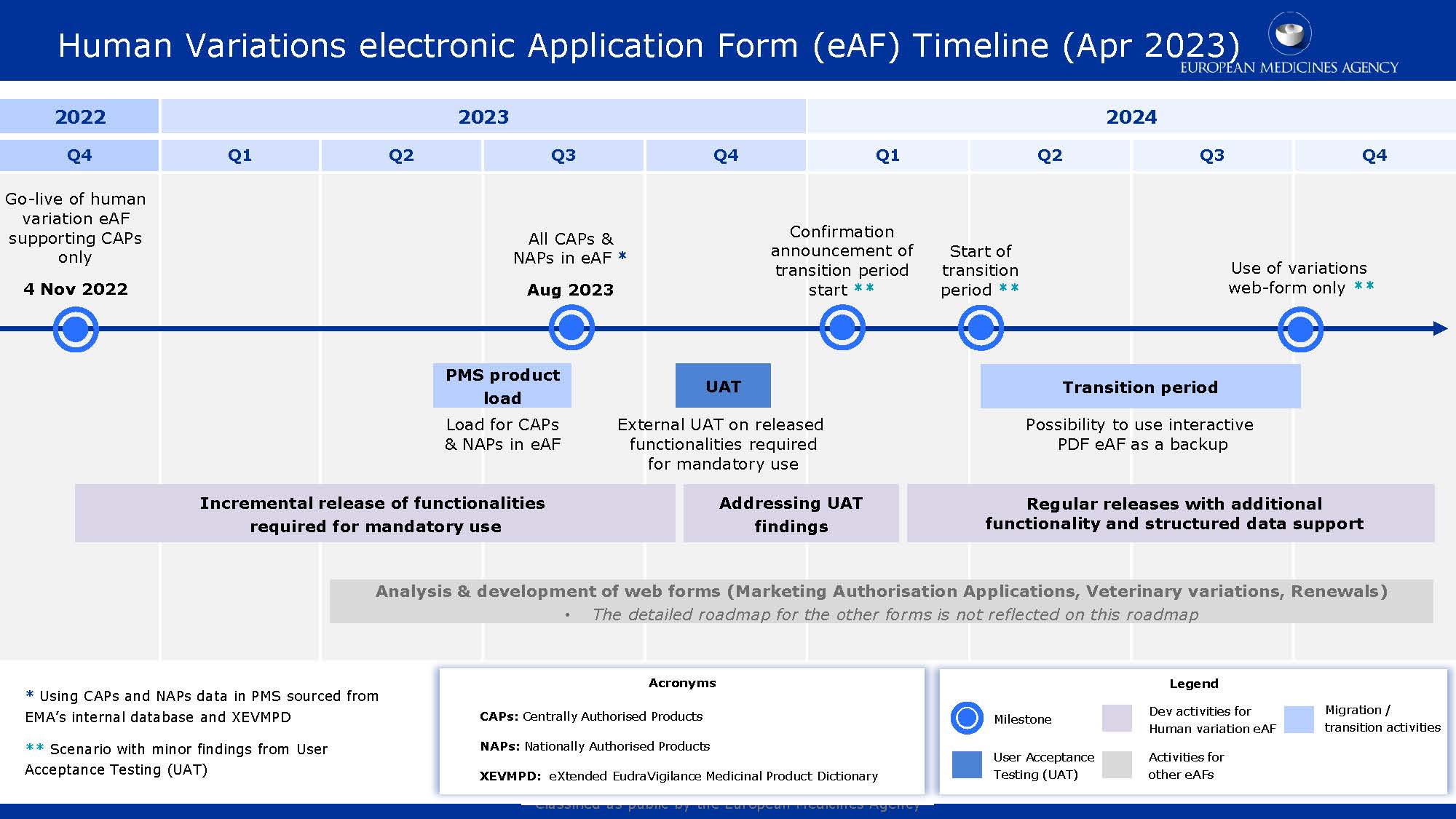
The PLM Portal Forum shows the eAFs implementation timeline. It will be as follows:
- June 2023: Data transfer begins. Centrally approved products (CAPs) and nationally approved products (NAPs) will be loaded into the PMS (via SIAMED and xEVMPD) and will be accessible in eAFs. The first NAPs can then be used in the web-based eAFs. After that, the NAPs will be gradually made available in the PLM portal.
- August 2023: Data transfer completion - all CAPs and NAPs are ready to apply the eAFs.
- November 2023: User Acceptance Test (UAT) with the latest version of the web-based eAFs to replace the pdf forms. This will initiate the switch to the new application forms.
- Confirmation of the changeover start date in Q1 2024. This will happen about two months before the scheduled start date and will be determined by the success of the UAT.
- Q2 2024: Begin of the six-month transition period for the required use of web-based eAFs for human medicine variations. This is contingent on the success of the UAT. The transition phase may begin sooner if practicable, subject to the two-month advance notice requirement.
(source: European Medicines Agency)
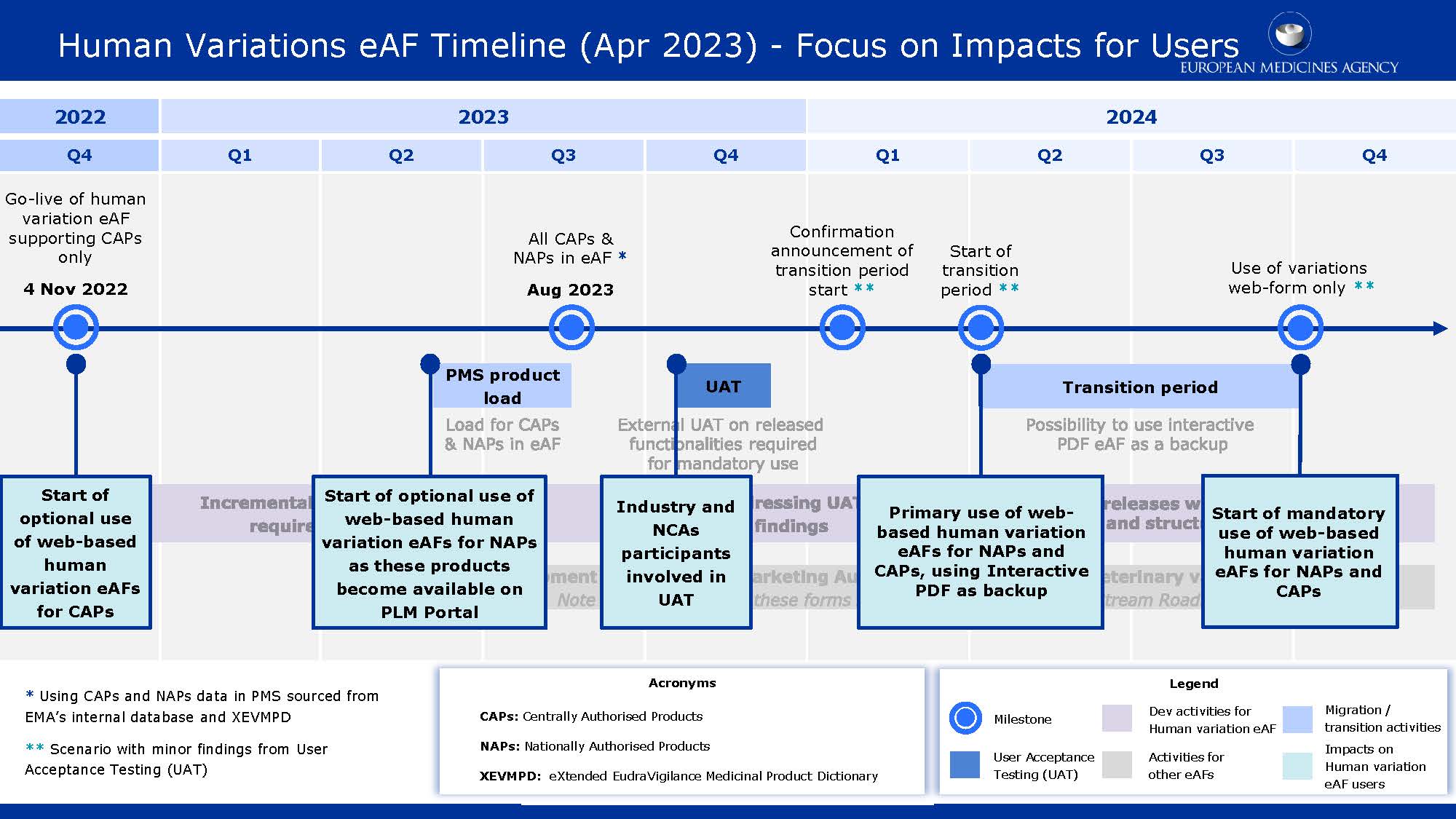
As extra information, a second version of the updated chronology discusses the consequences for users of eAFs in the various periods.
- The features necessary for mandatory eAF use will be deployed in stages. However, before the start of the UAT, every essential functionality will be offered in the PLM Portal.
- The web-based eAF will follow the existing data update process. Structured data will be introduced at some point afterward.
- In parallel, the capability of displaying migrated PMS product data in the PLM Portal product user interface is being developed; deployment dates will be disclosed later.
- The UAT's organizational details will be announced in due time.
(source: European Medicines Agency)
Use the following options to obtain additional information:
- eAF-PMS FAQs, which contain PMS-related questions and answers
- Q&A document for training
- Human Variations eAF go-live Q&A
- Human Variations eAF go-live training (2 February 2023)
If you like to learn more about eAF and its relation to DADI, please go to Where is DADI? - The new EMA eAF: Definition and Impact | EXTEDO
.png)
%20(2).png)

