%20(2).jpg?width=576&height=265&name=Updated%20notification%20method%20for%20QPPV%20and%20PSMF%20updates%20to%20MHRA%20(Replacing%20eCTD%20sequence%20submission%20requirement)%20(2).jpg)
Updated notification method for QPPV and PSMF updates to MHRA (Replacing eCTD sequence submission requirement)
The Medicines and Healthcare Products Regulatory Agency (MHRA) recently issued new guidelines outlining the steps for making specific changes to medical products. To support you in your daily operations, we summarized the key points for you.
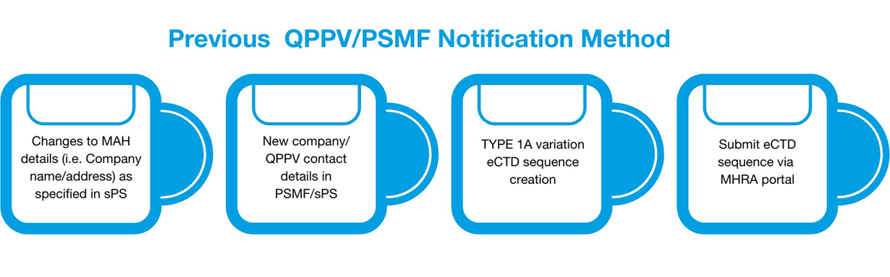
The current MHRA guidance effective as of 11 May 2023 states only sPS Update Notification is needed to replace the former condition for Type IA variation eCTD sequence for the following changes
· Changes to the Marketing Authorization Holder (MAH) details (i.e., Company name and address) as specified in the sPS
· The Qualified Person for Pharmacovigilance (QPPV) details (i.e., QPPV name and Contact address)
· The location in the UK where the PSMF can be accessed.
Notification of QPPV and PSMF details to XEVMPD
From 1 January 2021, for products in respect of Northern Ireland (UK-wide and Northern Ireland-only MAs), in addition to reporting the QPPV and PSMF details to the MHRA, you will need to submit information on the UK QPPV and the EU location of the UK PSMF to the database referred to in Article 57 of Regulation (EC) No 726/2004 (Article 57(2)).
How to avoid rejections of notification
Eventually, a notification will get rejected. To prevent this from happening, we recommend that you follow these recommendations:
Supply all required documentation as mentioned here regarding the “Guidance on qualified person responsible for pharmacovigilance (QPPV) including pharmacovigilance system master files (PSMF)” by the UK Government.
- Failing to supply all required documentation and information may lead to a rejection of the notification, which will require you to make a resubmission addressing all discrepancies.
Pro tip - Although the eCTD sequence for the same must not be submitted, the focus to avoid rejection should be on the organized assembly and submission of the documents mentioned in the link above.
Use the current and only method of notification (via the MHRA Submissions Portal)
Please note that any eCTD sequence submitted will be discarded as it is not required as part of this process.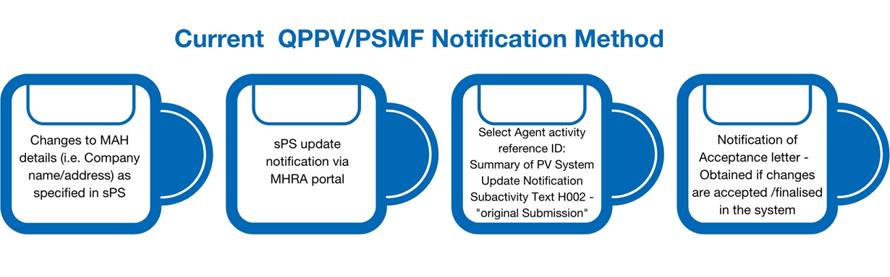
In conclusion, these updated notification methods for QPPV and PSMF are for notifying specific medication-related changes. However, to avoid rejections, keeping track of all the crucial information needed to make such a change is essential.
For more information on MHRA registration/usage and XEVMPD, please contact info@extedo.com.
About the writer
Priya Krishnan
Priya is a Junior Business Consultant at EXTEDO with a 3-year industry experience in Scientific Publishing and Regulatory Affairs. She has a double master's degree in Microbiology and MBA &E Life science Management, focusing on Regulatory Affairs.
.png)


.png)