.png?width=576&height=265&name=EXTEDO_Blog_6%20Steps%20to%20Your%20Successful%20eCTD%20Submission%20%20(1).png)
6 Steps to Your Successful First eCTD Submission - How to plan, prepare and publish
Preparing your first eCTD submission is a big deal! It marks a critical step in moving your product toward market approval. But let’s be honest - this process can feel like juggling flaming torches while walking a tightrope. As Helen Hayes, an American actress, once said: “The expert in anything was once a beginner.” Your first regulatory submission may feel daunting, but it’s the start of becoming an expert.
So, how do you go from chaos to control? In this blog, we have compiled the 6 most important steps to your successful first eCTD submission, share insider publishing tips, and show how EXTEDOpulse can turn a stressful experience into a structured, predictable workflow.
Why EXTEDOpulse Changes the Game
|
Why First Submissions Are So Hard
If you’ve ever asked, ‘Where do I even start?’, you’re not alone. Here are the usual pain points:
- Data everywhere: Product details scattered across spreadsheets and emails.
- Manual madness: Tracking timelines and documents in disconnected tools.
- Validation nightmares: Missing metadata or incorrect eCTD structure leading to agency rejections.
- Zero visibility: No clear picture of document readiness or review progress
- What, Where, and How: Uncertainty about which documents go into eCTD, what needs hyperlinking or metadata linking, and what should remain standalone.
Why These Steps Matter for Your Successful First eCTD Submission
Before diving into the process, it’s important to understand why following a structured approach is critical. A well-defined framework not only minimizes the risk of agency rejection but also streamlines workflows by reducing unnecessary rework. It ensures full compliance with ICH and regional standards, while providing transparency and control across teams. In short, structure is the foundation for efficiency, quality, and regulatory success.
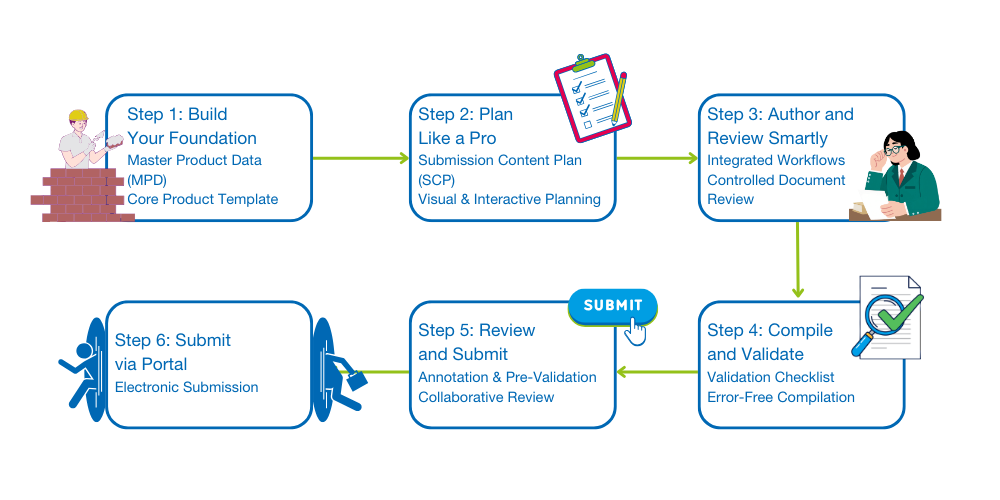
6 steps to building your first successful eCTD submission:
Step 1: Build Your Foundation
Start with Master Product Data (MPD) - your single source of truth for ingredients, dosage forms, packaging details, and indications.
How EXTEDOpulse helps: The Registration Management Hub empowers you to build a reusable Core Product template. No more copy-paste chaos - just clean, consistent data across all regions.
Step 2: Plan Like a Pro
Don’t wait until the last minute to figure out what documents you need. Use a Submission Content Plan (SCP) to list all required documents by eCTD section, assign responsibilities, and track readiness.
The EXTEDOpulse advantage: The Document Management Hub powered by CARA makes SCP planning visual and interactive. You can see what’s missing at a glance - no more guessing games.
Step 3: Author and Review Smartly
Documents are the backbone of your submission. Common mistakes include using outdated templates, skipping controlled review workflows, and losing track of versions. Consider using our pre-written eCTDtemplates to speed up content creation and reduce rework.
How EXTEDOpulse makes it easy: Integrated workflows keep every document reviewed, approved, and connected to your Submission Content Plan - so when it’s time to compile, everything falls into place effortlessly.
Step 4: Compile and Validate Without Tears
Validation errors can derail timelines. During eCTD validation, issues are classified as errors, warnings, or best practice notices. Errors are critical and must be resolved before submission, as they can lead to rejection. Warnings are less severe but may prompt questions from health authorities, while best practice notices are recommendations to improve quality and consistency.
Among these, errors require the highest priority. To minimize risk, use the following checklist before publishing:
- Are all metadata fields complete?
- Do document names follow eCTD rules?
- Does the sequence pass regional validation criteria?
What EXTEDOpulse delivers: With Submission Management Hub, your planned documents flow seamlessly into a structured submission sequence, ready for validation.
Pro Tips for First-Time Submitters
|
Step 5: Review and Submit
Once compiled, review your sequence in a structured way: Use annotation tools for feedback, export an annotation report for authors, and pre-validate before submission to avoid agency rejections.
The EXTEDOpulse bonus: Additionally, the Submission Management Hub turns review and publishing into a collaborative experience.
Step 6: Submit via the Correct Agency Portal
Deliver your validated submission through the appropriate portal - EMA, US FDA, or others - while meeting all delivery guidelines. With EXTEDO’s publishing experts and EXTEDOpulse, submission becomes accurate, timely, and stress-free.
What Does It Take to Go From First Submission to Publishing Excellence?Submitting for the first time? Start strong with practical tips and consider leveraging expert services backed by advanced software for a smoother experience. Combining guidance with technology ensures accuracy and efficiency from day one. |
A smooth submission process follows six steps: Start by building a strong foundation with Master Product Data as your single source of truth. Then plan ahead with a clear Submission Content Plan that lists documents, assigns responsibilities, and tracks readiness. Author and review using controlled workflows to avoid outdated templates and version confusion. Next, compile documents into a structured sequence and validate thoroughly to prevent errors and delays. Review collaboratively, apply feedback, and pre-validate before submission. Finally, deliver through the correct agency portal, meeting all regional guidelines for accuracy and timeliness. These steps turn a complex process into an organized, predictable path to success.
Ready to make your first submission a success? Reach out to info@extedo.com!
Author:
.png?width=200&height=200&name=Priya%20(2).png) |
Priya Krishnan |
.png)


.png)