

Effortless Safety Case Management
Use SafetyEasy™ to transform the complexity of managing increasing volumes of safety cases, regulatory requirements, and data into a streamlined, effortless process.
Ensure compliance while increasing efficiency and reducing costs
Easily track safety activities and submission deadlines
Assure data quality via business, technical and internal validation
How long is your backlog of safety cases?
Managing an increasing number of safety cases can quickly get overwhelming without the right tools.
- I can't keep up with the increasing number of cases
- I am struggling to cope with additional safety requirements
- I am losing track of my follow-up activities
- I am struggling to keep an oversight of my reporting timelines for ICSRs
- I am unable to efficiently handle different types of reports in my system
- I am worried about handling clinical trial cases in a compliant and efficient way
- I am struggling to generate different types of forms, line listings and summary tabulations
- I am unsure about how I can do signal detection in an efficient way


Ensuring patient safety should be effortless.
Compliance with Regulations
SafetyEasy™ ensures compliance with various drug safety regulations, including E2B(R3), HL7 eMDR, and future standards. It facilitates the reporting and management of adverse events and supports the generation of essential regulatory documentation.
Cost-Effectiveness
The platform is designed to reduce operational costs. It offers a single database for safety data and operates as a cloud-based service, eliminating the need for extensive customization or training.
Efficiency Enhancement
SafetyEasy™ increases operational efficiency through its E2B gateway solution and bi-directional data exchange capabilities. It also offers API connections for seamless data and functionality integration.
Workflow Optimization
The software streamlines workflow management, allowing users to track and monitor project statuses. Features like email notifications and online dashboards ensure timely completion of tasks and compliance with submission deadlines.
.png)


"SafetyEasy has proven to be a highly effective solution. It has streamlined our pharmacovigilance processes, significantly reducing manual tasks and minimizing the risk of errors. Our team is delighted with the solution and services provided."
Your plan to effortless compliance
Book a demo
Our experts will show you how SafetyEasy™ simplifies your safety case management.
Create a plan
We discuss your safety process and outline an approach to simplify your case management.
Manage safety cases effortlessly
With SafetyEasy™, you know that your safety cases are being managed accurately and efficiently.
Streamline your safety case management
With an ever-increasing amount of safety cases, teams can quickly become overloaded and miss important details and deadlines.
SafetyEasy™ provides a way to effortlessly manage safety cases and vigilance data, enabling you to compliantly process more cases, faster.


View the product information factsheet
Ensuring compliance with E2B(R3) and HL7 eMDR safety regulations
Built specifically to support the E2B(R3) EudraVigilance system and MedDRA coding standards, SafetyEasy™ handles the reporting and management of all serious and non-serious adverse events. Its future-proof approach is able to generate PSUR, PBRER, and DSUR documentation and is ready for forth-coming standards such as IDMP. It also supports eMDR XML file creation. Through an EMA certified gateway, SafetyEasy™ provides you with a direct link to the regulatory authorities, eliminating the need for manual submission of reports.
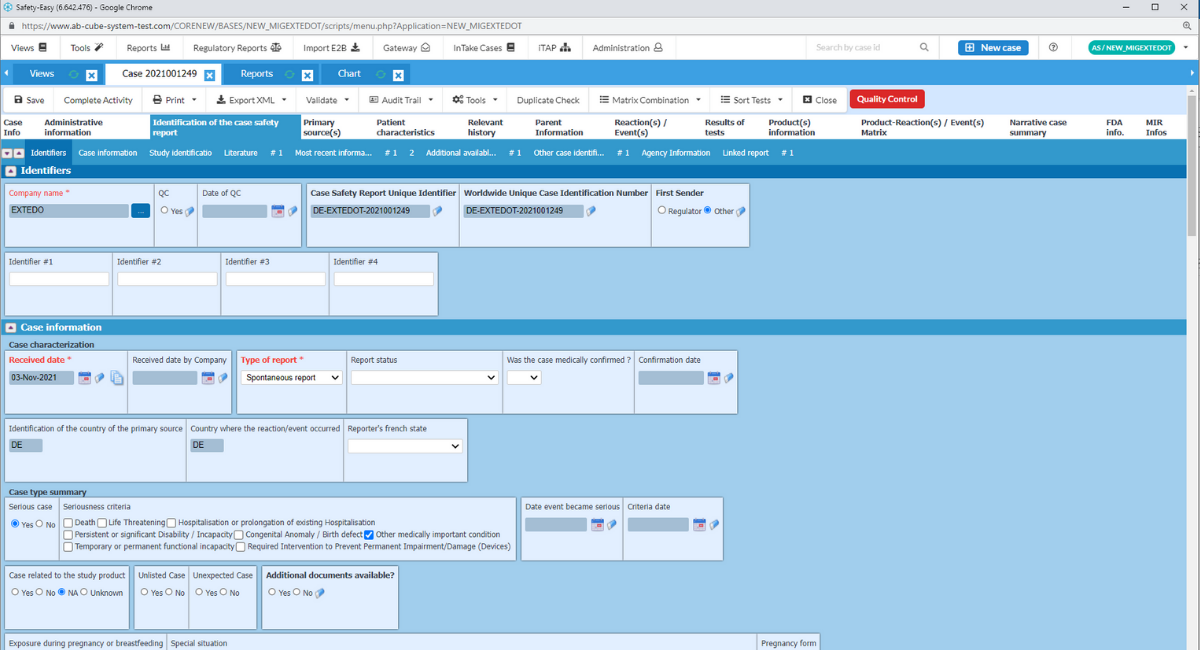
Streamline workflows, optimize your productivity
SafetyEasy™ also enables you to readily track and monitor the status of workflows with every project in your organisation. Through email notifications and online dashboards, SafetyEasy™ provides users with reminders about imminent activities they need to perform. Now, you can ensure that your team members are staying productive and in the know. This guarantees submission deadlines are met, and other legal obligations are never overlooked again.
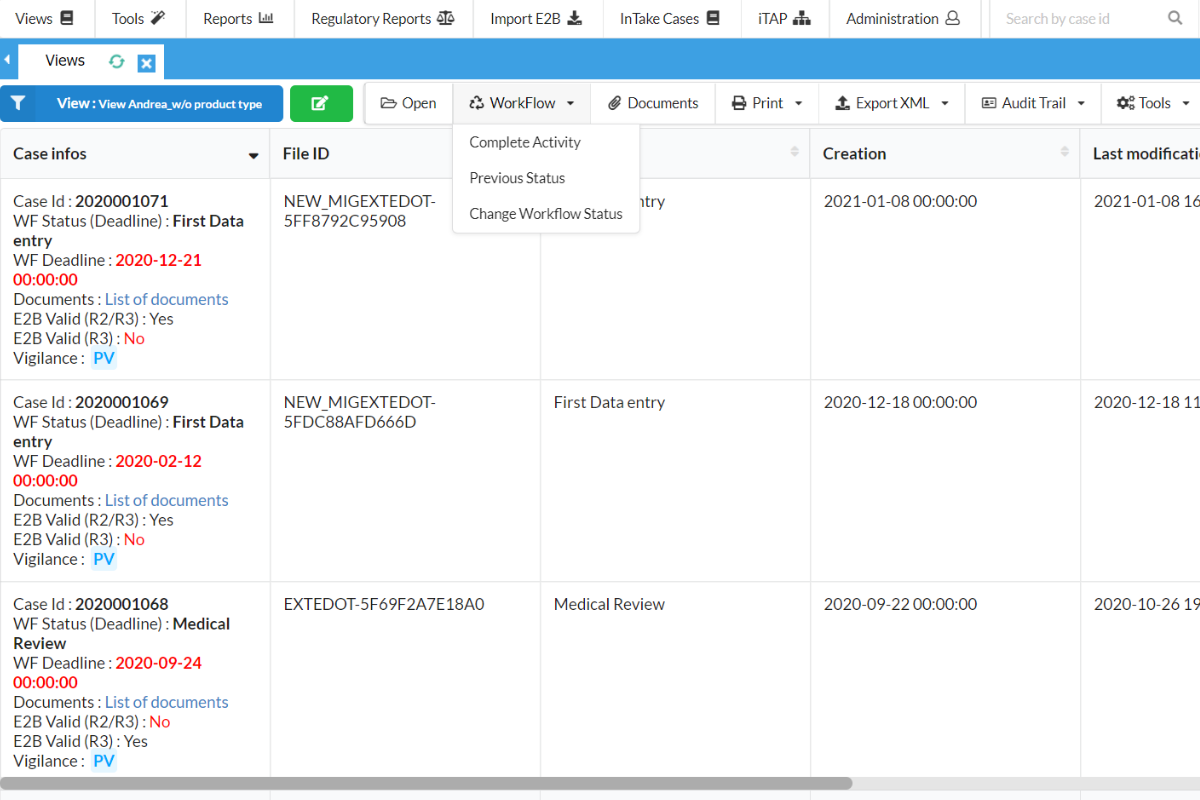
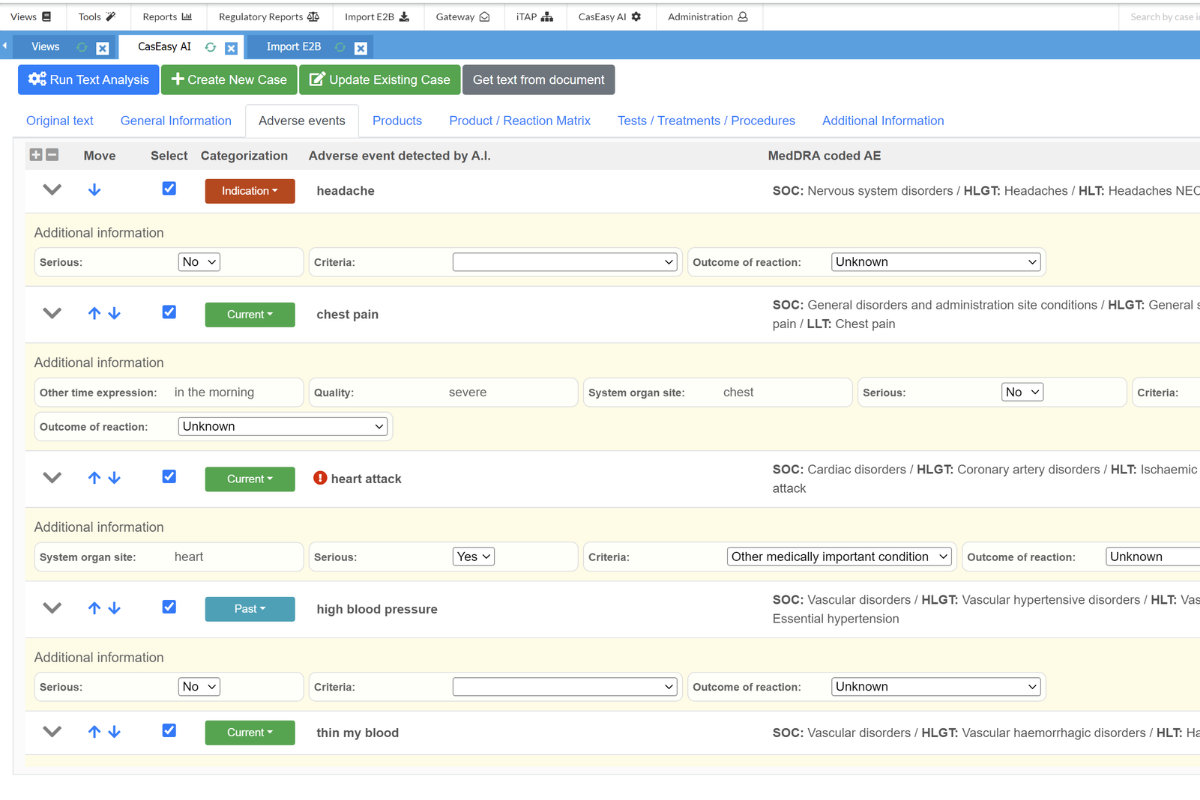
Enhanced Pharmacovigilance with Artificial Intelligence (AI)
The CasEasy AI module leverages advanced Natural Language Processing (NLP) to streamline case creation. With CasEasy AI, imported or added ICSR verbatim text can automatically be converted into a case in SafetyEasy™, significantly reducing manual case creation times. It supports importing files in PDF, JPEG, and PNG formats, even handwritten documents if needed. This module also uses AI to suggest Adverse Events (MedDRA-coded) and flag potential serious cases.
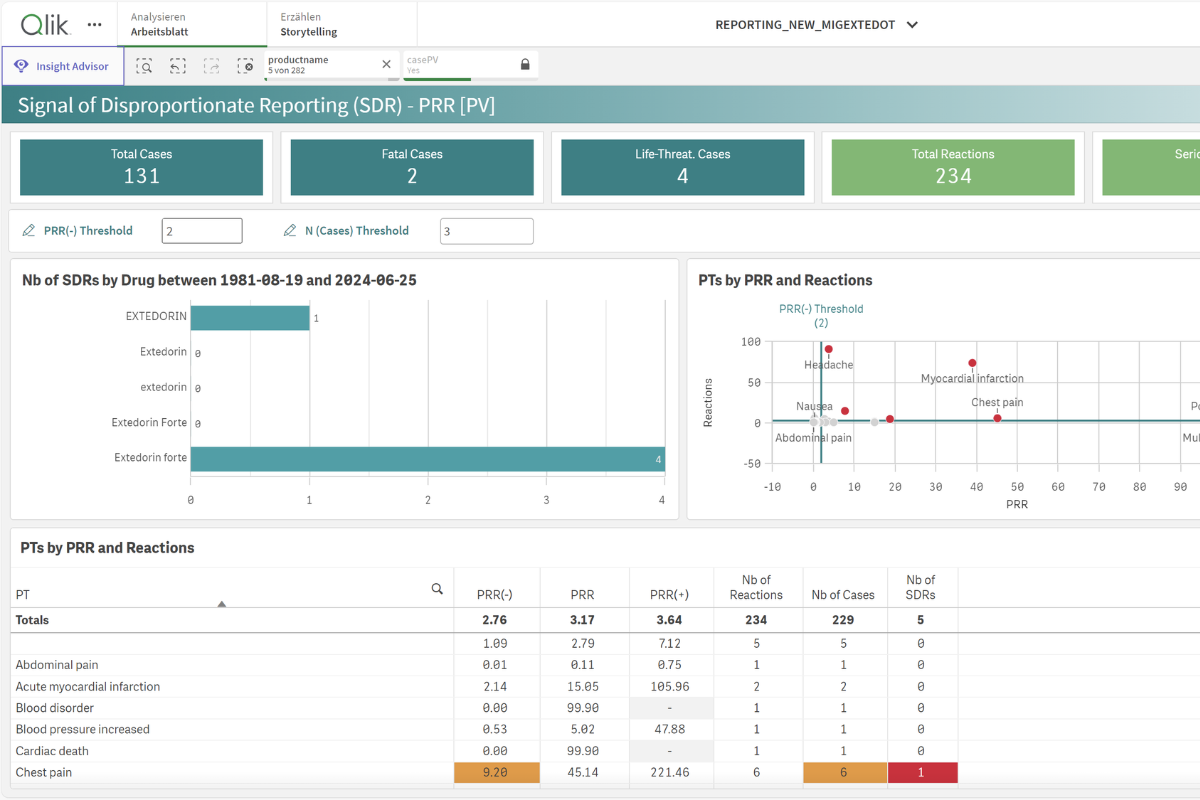
Enhanced Compliance and Analysis with the Business Intelligence (BI) Module
Furthermore, the Business Intelligence module provides a 360° dynamic view of your scientific data, which enhances case analysis and improves the detection of safety signals. This powerful BI module is powered by Qlik Sense technology, which is also used by the US FDA and helps to boost your company’s efficiency and compliance. Compliance levels increase and reporting and KPI follow-up speed up.
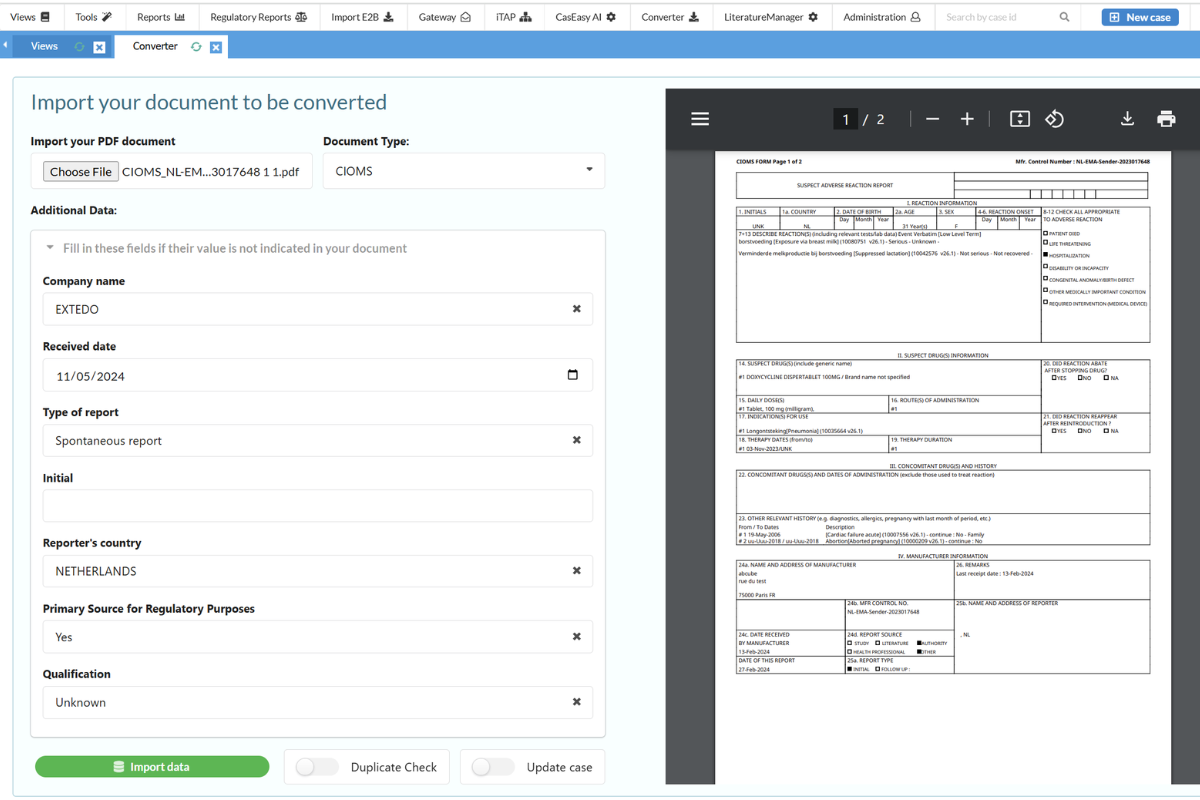
Streamline Your Workflow with the SafetyEasy™ Converter
The SafetyEasy™ Converter module is designed to save your team valuable time when entering cases into the system. By incorporating Optical Character Recognition (OCR), this AI-powered module automatically extracts and processes case information from uploaded forms, such as CIOMS, Medwatch, or SAE. The Converter not only reduces case intake time by up to 70% but also ensures that all required data is accurately captured and entered directly into SafetyEasy™.
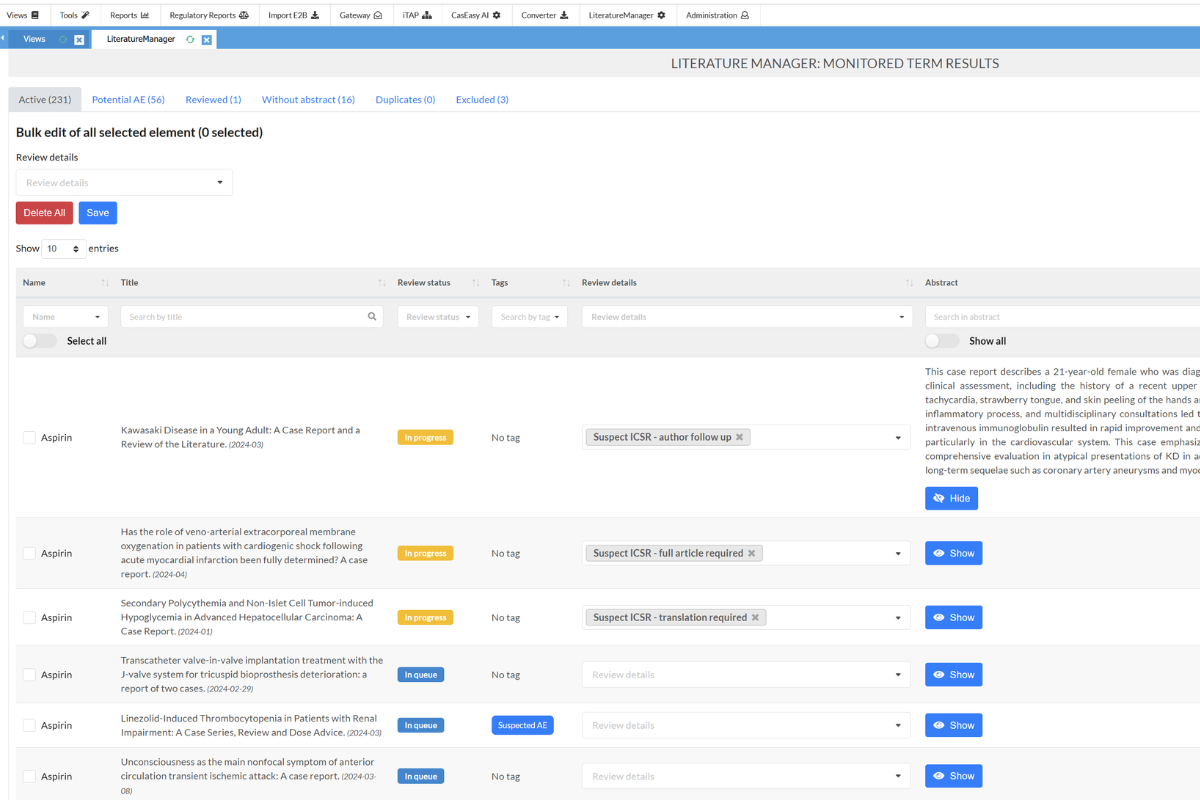
Optimize Surveillance with the SafetyEasy™ Literature Manager
The Literature Manager module optimizes surveillance by saving your team time on article screening. This comprehensive solution automatically connects to PubMed, helping you find, analyze, review, and create potential cases. The Literature Manager supports an unlimited number of products and uses advanced Artificial Intelligence to triage and sort only potential pharmacovigilance cases, significantly reducing screening volumes. Abstracts are processed through CasEasy AI, seamlessly creating cases in SafetyEasy™.
Cloud-based pharmacovigilance, medical device vigilance, cosmetovigilance and nutrivigilance software-as-a-service
As a secure, cloud-based service, SafetyEasy™ is lightning quick to implement and requires no customization. In many instances, SafetyEasy™ can be configured and validated within two weeks. Its simple, intuitive, and user-friendly interface speeds user adoption and eliminates the need for extensive training. It is a complete, out-of-the-box solution for health science organisations of any size, location and speciality.

Used worldwide for guaranteed compliance
Used by more than 300 organizations across 90 countries, SafetyEasy™ is the simplest and most cost-effective way to ensure effortless compliance with current and future drug safety regulations. With ICH, EMA, FDA, EU GMP Annex 11, US FDA 21 CFR part 11, and EMA’s Good Pharmacovigilance Practice (GVP) guidelines, SafetyEasy™ is compliant with many regulations and directives from around the world.
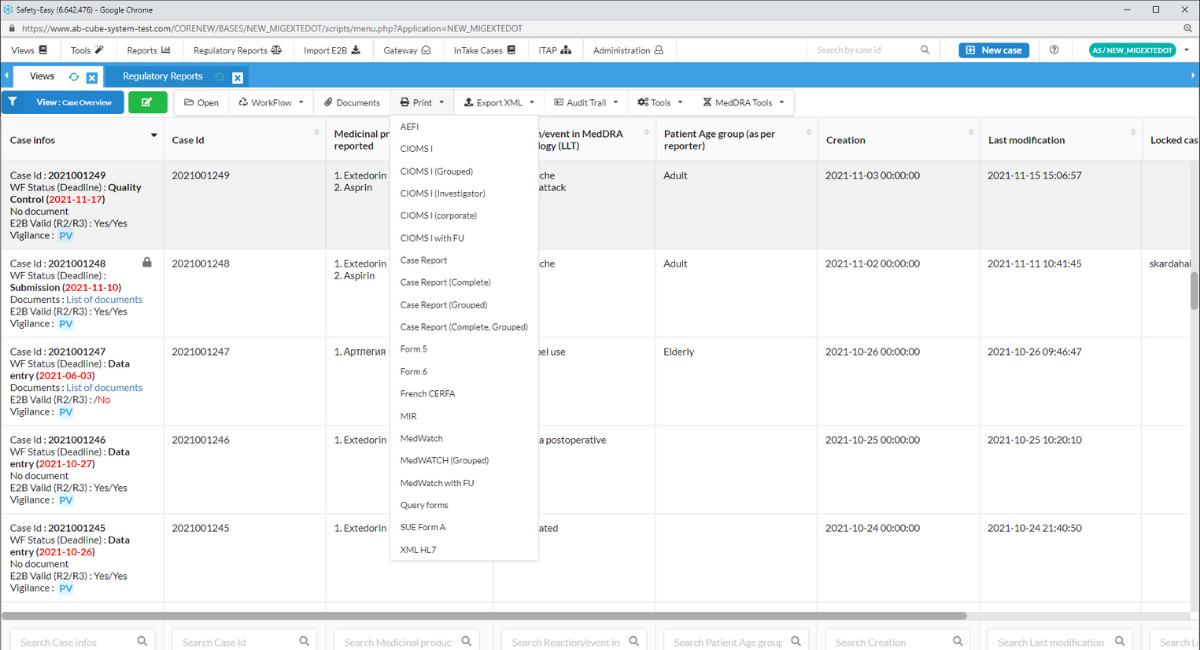
Triage and assessment of ICSRs in E2B(R3) with iTAP
iTAP is a fast, efficient solution created to help you with the triage and assessment of your ICSRs in the E2B(R3) format with customizable filters. L2A and/or MLM cases are retrieved from the Eudravigilance database and assessed enabling you to select relevant cases for your product portfolio. Every decision for each ICSR you make is tracked by iTAP so you can upload relevant E2B xml files with SafetyEasy™ directly to your database quickly and easily.

Some of our Customers




Contact us
Pharmacovigilance Services
EXTEDO also offers Pharmacovigilance Business Process consulting services, which are tailored specifically to your needs. Based on many years of experience working with drug safety rules and regulations, EXTEDO´s team will help you to identify gaps in your pharmacovigilance processes and help you to develop and implement appropriate strategies PSMF to resolve them.
Education & Training Services
To ensure you get the most out of your purchased solution, we offer detailed training for each product within the EXTEDOpulse solution portfolio. Training sessions are tailored to your individual needs and cover a broad range of technical and regulatory topics. Designed to educate you on how to utilize your EXTEDO solution, our training sessions are conducted either in-house or onsite.
Technical Consulting
Purchasing a new EXTEDO application is the first step to streamlining business and regulatory processes within your organization. However, ensuring correct installation, implementation and integration is a crucial step in the process of deploying your new solution.


