

DOCmanager Module for Global eSubmission Management
Reducing the effort of managing multi-region product dossiers.
Reduce errors and increase productivity through advanced automation
Reduces update times for variations and streamlines the process of managing submissions
Creation and efficient maintenance of many child dossiers based on one parent dossier. Any changes there are automatically inherited by the child dossiers
The process of marketing pharmaceutical products in multiple countries comes with its own set of complex challenges.

Selling a product or ingredient to different customers operating in separate countries and/or regions means that you have to prepare and manage multiple eSubmissions that are compliant with the regulatory requirements of each particular country/region.
With submission requirements differing from region to region, the process of creating and managing regulatory submissions on a global scale can be complex and costly. While much of the information required within each submission is universal, it is common that certain elements apply only to specific regions or countries. In addition, the lifecycle of a submission may vary from region to region, meaning that documents may need to be independently replaced, added, or deleted across many different countries.
Active pharmaceutical ingredient (API) manufacturers face a similar challenge when preparing submissions for different authorities in various countries. Selling just one active substance to 5 customers that operate in 5 countries means managing 25 dossiers in parallel. API dossiers, such as those conforming to ASMF and DMF submission standards, can evolve into different versions for internal use and customer use, thus handling multiple dossiers becomes even more complex task.

Request your personal EXTEDO Software Demo now!
Streamlining global submissions with DOCmanager
EXTEDO’s DOCmanager, an extension to eCTDmanager, helps you address the challenges associated with creating and managing regulatory submissions across multiple regions. Through the use of a parent-child dossier concept, DOCmanager enables you to readily generate templates for multiple submissions through simple country-level changes whilst keeping common data managed at a product level. You can reuse the content for dossiers based on different submissions scenarios, thus reducing the efforts associated with compiling and maintaining multi-region product dossiers.
Any changes you make to the parent dossier are automatically inherited by the child dossier, thus eliminating the need for revalidation. DOCmanager supports eCTD submissions according to the European MRP and DCP procedures, thus enabling you to update individual submissions or create group updates. In addition, DOCmanager enables you to create inter- and intra-document hyperlinks as well as print publishing templates that are maintained on the parent dossier and can be passed on to child dossiers. This allows you to manage changes at any level by simply inserting a single change and then passing it on to all child dossiers.
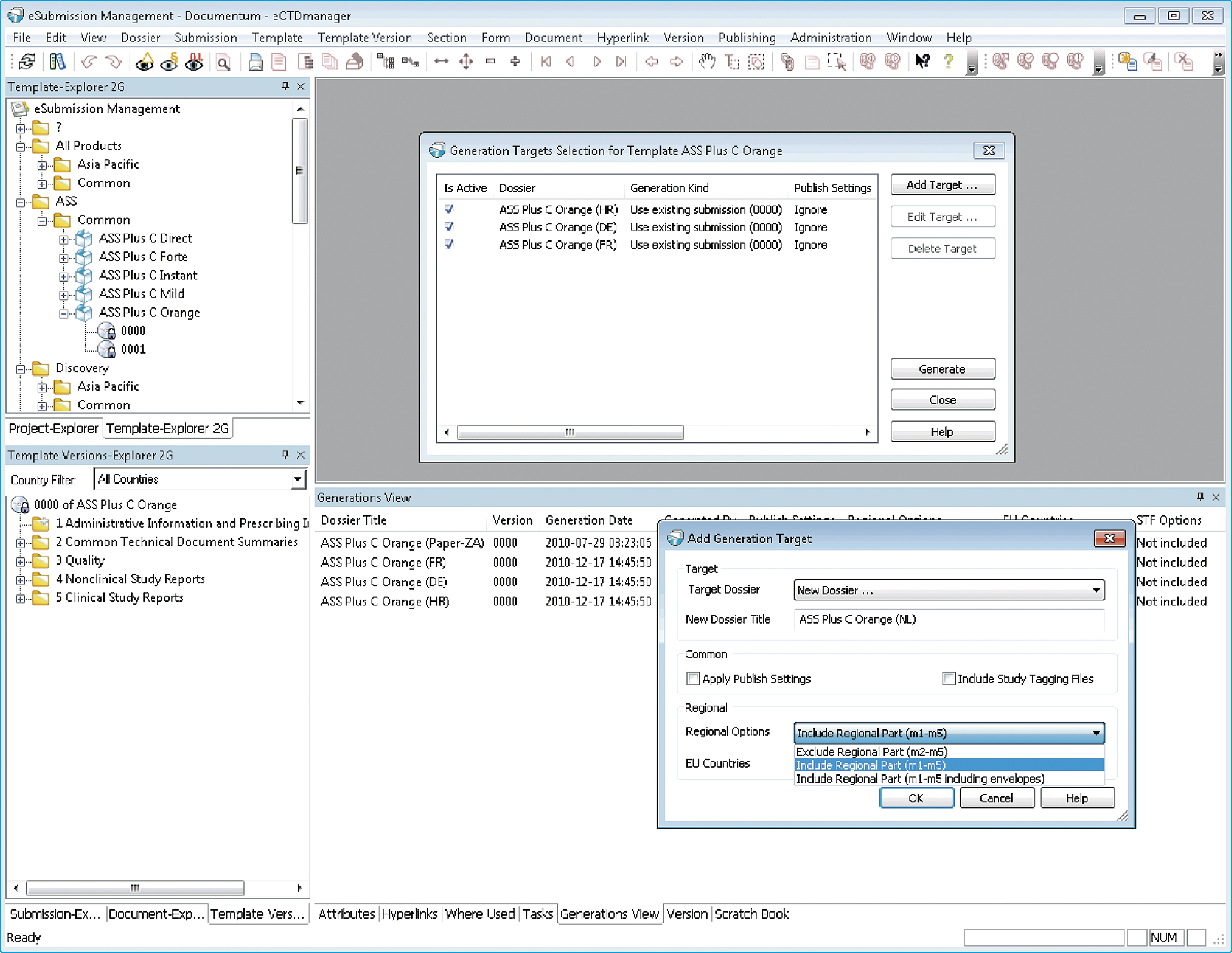
Efficiently manage regulatory submissions in multiple regions
By using Document Queries, you can search for documents with specific metadata and place these documents automatically into the eCTD structure within eCTDmanager. Used by SMBs and multinational companies around the globe, DOCmanager is the proven solution for efficiently managing regulatory submissions in multiple regions. It saves time, reduces your costs, and eliminates the errors associated with traditional manual dossier management techniques.


“With eCTDmanager and DOCmanager we have a perfect system in place that allows us to efficiently plan and manage submissions and be compliant with regulatory requirements in a wide variety of countries.”
Director of Regulatory Informatics & Submission Management
Your plan to effortless compliance
Schedule a call
We’ll discuss your goals and uncover your challenges with viewing and reviewing electronic submissions.
Get a free consultation
Our experienced business and technical team will outline a solution to solve your challenges.
Ensure effortless submission compliance with global regulations
Ensure compliance and manage your eSubmissions across multiple countries, requirements and conditions.


