
White paper:
Everything you need
to know about eCTD
Implementing eCTD can be daunting due to its stringent and varied global standards. It often leaves regulatory teams feeling...
- Frustrated with adapting existing processes to fit the eCTD
- Confused by the technical specifications and requirements
- Concerned about the high risk of non-compliance and potential rejections
- Stressed by the steep learning curve for new staff or team members
We understand these pressures and are here to helping you overcome them.
Download our white paper now to discover strategies and insights that can simplify your eCTD implementation process.
Download the white paper
Master the eCTD requirements
Get a better understanding of the eCTD process with our detailed white paper. This document covers advanced strategies, updates, and ways to optimize your regulatory submission processes.
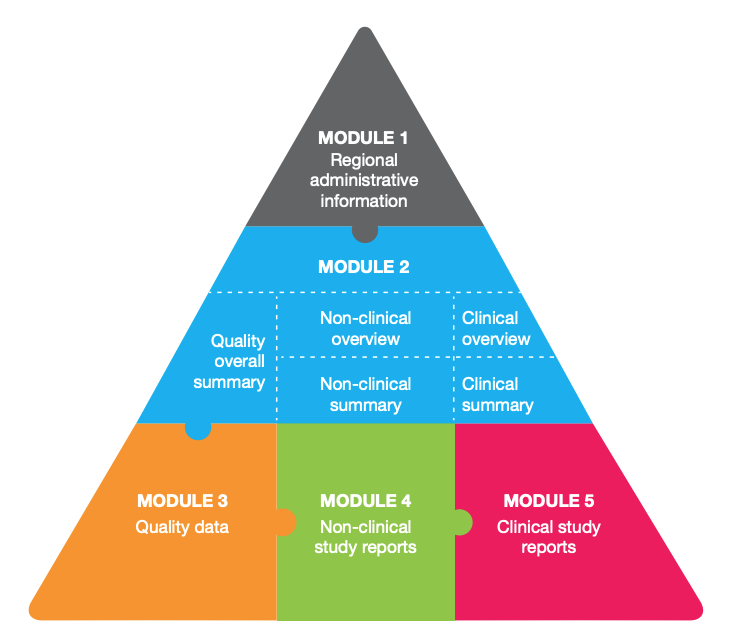
Advanced module management
Gain a deeper understanding of each module's role and how to maximize their effectiveness in your submissions.
eCTD around the world
Explore how eCTD standards are applied across different regions and the implications for global compliance.
Planning your eCTD project
Understand the critical steps for successful eCTD implementation, from readiness assessment to system validation.
Essential questions to ask eCTD software vendors
Arm yourself with key questions that will help you choose the right software solutions and ensure seamless integration.
Fill in the form to deepen your expertise and stay ahead in the dynamic field of regulatory submissions.
Our Customers





