EURSvalidator - EXTEDO's NeeS and eCTD Validator
Validate the technical compliance of your eCTD and NeeS submissions against regional requirements before submitting them to agencies worldwide.
Ensure compliance with regulatory requirements worldwide
Up-to-date: Latest validation sets provided in timely manner
Validation technology trusted by over 35 regulatory authorities globally
Sending non-compliant submissions causes unnecessary delays.
Your submissions may have passed validation internally, but when they fail at the regulatory agency, fixing issues can be time-consuming.
- I am uncertain about regional variations in submission standards
- I can’t trust the results from our existing internal validation tools
- I am unsure why my submission was invalid or how to fix it
- I get overwhelmed by ever-changing global submission requirements


How we solve the problem
Most accurate eCTD & NeeS validator
EURSvalidator ensures the highest possible accuracy when validating your eCTD and NeeS submissions. It is used by over 35 regulatory authorities worldwide to validate incoming submissions.
Up-to-date validation sets - guaranteed
EXTEDO works closely with regulatory authorities worldwide.
We guarantee that all relevant validation sets are included in each regional package and that validation sets are always up-to-date.
Validate multiple submissions simultaneously
Start the validation of all pending dossiers simultaneously. All pending dossiers and submissions that have not yet been validated will be validated simultaneously.
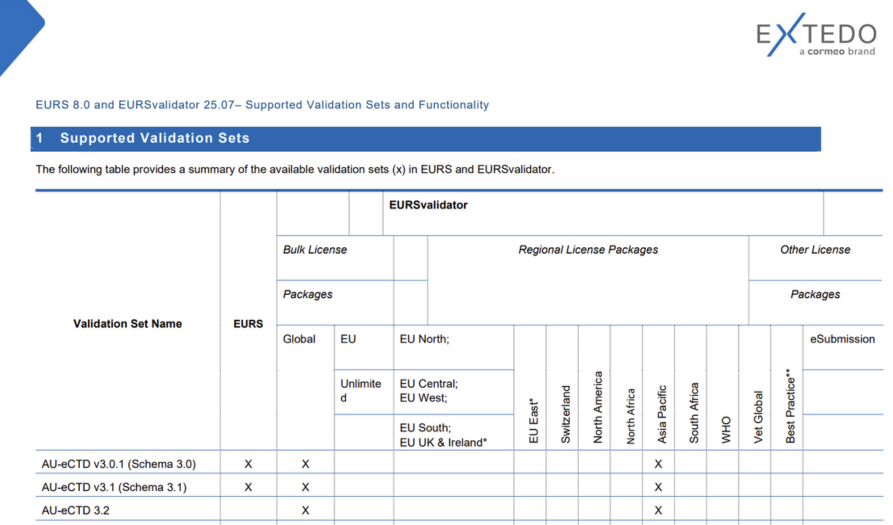
Regional validation packages
Regional subscriptions are available, including validation sets for the country or region you want to file your submission within.
Different validation set packages can be combined within a single subscription.



"EXTEDO is a great example of a company that really understands the requirements of the market."

Request your personal EXTEDO Software Demo now!
Your plan to effortless compliance
See how it works
Watch the product video and see how to simplify validation of your regulatory submissions.
Get a quote
Provide us with information about your validation needs, and we will send you a personalized offer.
Pass validation
Relax as your submissions pass agency technical validation first time.
Make regulatory submissions to authorities around the world without the worry of technical errors
Validating that electronic submissions are compliant with regulatory technical standards worldwide can be complex without a reliable tool.
As the validation tool used by the authorities, EURSvalidator ensures compliance first time, speeding up your submission processes and getting your products to market faster.

Some of our Customers